FERRIC CITRATE AND CHRONIC KIDNEY DISEASE IN CHILDREN
We are enrolling children with chronic kidney disease mineral bone disorder(CKD-MBD) for a randomized control study

What is the purpose of this study?
- The purpose of this NIH-funded placebo-controlled clinical trial is to determine how well ferric citrate will lower FGF23, correct anemia and iron levels in the blood, and maintain normal levels of phosphate in children with CKD.
- Chronic Kidney Disease (CKD) is a condition in which the kidney function is decreased.
- Fibroblast Growth Factor 23 (FGF23) is a hormone that regulates blood phosphate levels. High FGF23 levels are linked with worsening of kidney, bone & heart health.
- Low levels of hemoglobin, called anemia, are common in CKD. Increasing iron stores in the body can help raise hemoglobin levels.
What is Ferric Citrate?
- Ferric Citrate is an oral medication that decreases the absorption of dietary phosphate by the gut, lowers FGF23 levels and raises iron stores in the body.
- Ferric citrate is approved by the FDA for use in adults with CKD.
How Does Ferric Citrate Work?
Ferric citrate helps to lower the amount of phosphate in your blood.
- Take with meals
- After ferric citrate enters your gut, the tablet breaks apart and releases iron
- Iron attaches to the phosphate that was in your food, where it stays in your intestines, gets moved to your colon, and eventually is eliminated from your body when you take a bowel movement
- Leftover iron may be absorbed into your body
- Age 6-18 years
- Stage 3 or 4 CKD
- Normal phosphate levels
- No prior transplant
- Able to swallow tablets
- Eat at least two meals per day
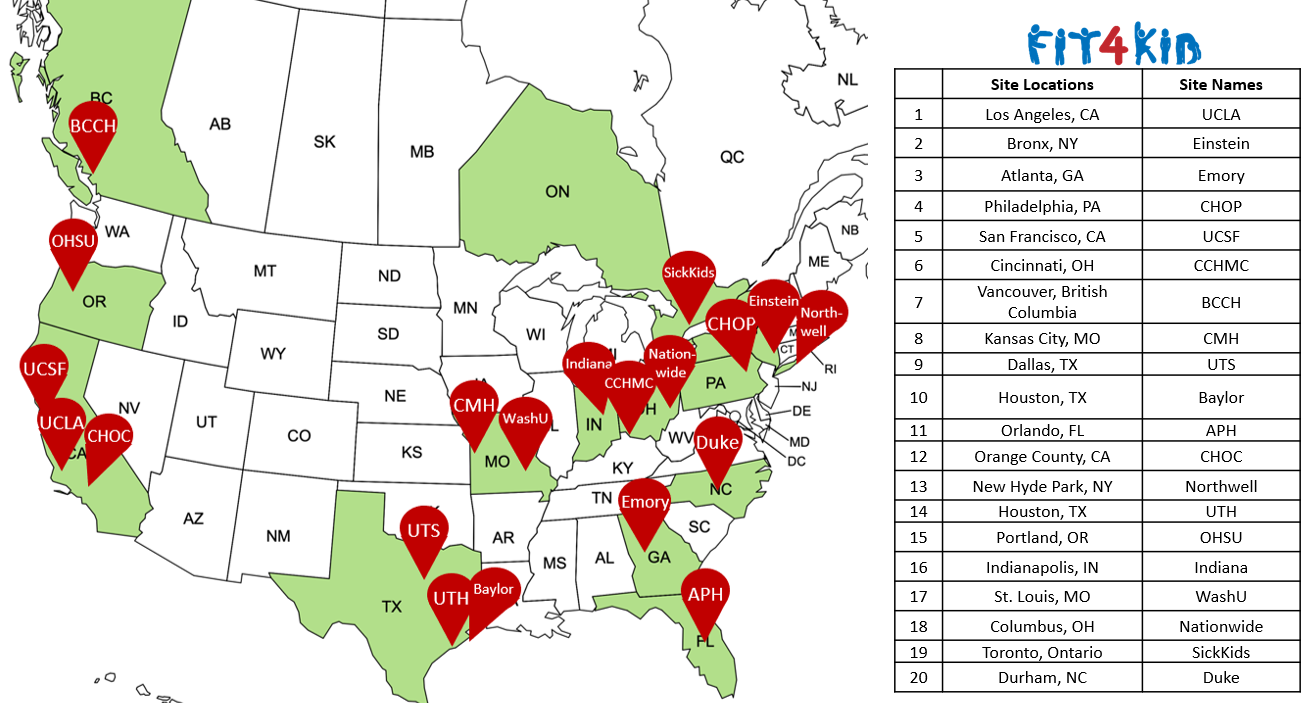
- Goal: 160 participants from 20 hospitals in North America
- Participants will be randomized to receive ferric citrate or placebo
- Study duration: 12 months (6 visits)
- Participants will be asked to give blood and urine samples and complete surveys about symptoms
Study Information
Chronic Kidney Disease (CKD)is a condition in which the kidney function is diminished.
Phosphate is a mineral found in the bones in your body. People get phosphate from the food that they eat. Kidneys remove excess phosphate from the blood.
Fibroblast Growth Factor (FGF23) is a hormone that regulates blood phosphate levels by “telling” the kidneys how much phosphate to get rid of in the urine.
As kidney function gets worse, FGF23 levels get higher. High FGF23 levels are linked with kidney, bone, and heart problems.
Low levels of hemoglobin (called anemia) is also common in children with CKD. Increasing iron stores in the body may help to raise hemoglobin levels.
What is the Purpose of the Study
To determine how well ferric citrate lowers FGF23 levels, corrects anemia and iron levels in the blood, and keeps phosphorus levels normal in children with CKD.
This medicine is approved in adults with CKD but has not yet been studied in children.
Children will be randomized to receive either ferric citrate or placebo.
The study plans to enroll 160 patients across North America.
Am I Eligible to Participate?
- Age 6 – 18 years old
- Stage 3 or 4 chronic kidney disease
- Normal phosphate levels
- No prior transplant
- Able to swallow tablets
- Able to eat at least 2 meals a day
What Does the Study Involve?
- 12 month study
- 6 site study visits
- 9 phone call visits
- Participants will be asked to give blood and urine samples and to complete surveys about symptoms
What Are Possible Risks?
- Low phosphate levels
- GI symptoms like discolored stools, diarrhea, constipation, nausea, or vomiting
Supported by grant UO1DK 122013 from the National Institute of Diabetes and Digestive and Kidney Diseases